Abstract
Introduction Patients with hereditary hemochromatosis (HH) and non-transfusion-dependent hereditary anemia (HA NTD) both express low hepcidin levels, leading to increased intestinal iron absorption and, ultimately, predominantly parenchymal iron overload. Knowledge about iron absorption in humans stems from iron absorption or utilization studies with radio-labeled iron performed in the 60-70s from the last century. Here, we present unique data of combined absorption and utilization studies in a large cohort of patients with primary and secondary hemochromatosis.
Methods We retrospectively analyzed the data from iron absorption and kinetics studies performed from 1972 until 1994 as part of routine clinical practice in patients with iron-related health problems at the University Medical Center Utrecht, the Netherlands. A radioactive tracer dose of oral (1 mg) 59Fe with 51Cr as non-absorbable indicator, or intravenous (10 µCi) 59Fe was administered. Radio-activity was measured with a whole-body counter to assess absorption and with a gamma-counter to determine radio-activity in peripheral blood samples to calculate the amount of iron utilized for red blood cell (RBC) production.
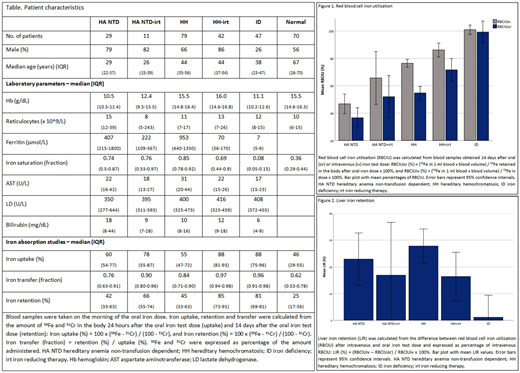
Main findings Iron absorption was analyzed in 6 distinct groups with and without iron overload and iron reducing therapy (table). Iron uptake is the percentage of the iron test dose taken up by enterocytes, retention the percentage retained in the body 14 days after ingestion, and transfer the fraction of iron taken up that is retained in the body. Iron uptake, transfer and retention were significantly higher in patients with treated and untreated HH and iron deficiency (ID) compared with healthy controls (p<0.01). Notably, uptake, transfer and retention were also significantly higher in the analyses of patients with treated or untreated HA NTD (including 19 congenital sideroblastic anemia, 6 hereditary spherocytosis, 5 congenital dyserythropoietic anemia, 3 non-transfusion dependent thalassemia, 4 Hb Adana, 2 hexokinase deficiency and 1 PKD) than in analyses of healthy controls (p<0.01). Next, iron retention was used to calculate the percentage of iron utilized for RBC production after 14 days. Mean percentages of RBC iron utilization (RBCIU) after an oral iron test dose were 37% (SD 17%) in untreated HA NTD, 53% (SD 19%) in treated HA NTD, 55% (SD 20%) in untreated HH, 70% (SD 22%) in treated HH, and 99% (SD 22%) in ID patients. Surprisingly, RBCIU was lower after oral than after intravenous iron in patients with HA NTD or HH (figure 1). The difference between oral and intravenous RBCIU was expressed as percentage of intravenous RBCIU, and denominated as the LIR (liver iron retention). The LIR had a mean value of 28% (SD 26%) in untreated HH, 23% (SD 24%) in untreated HA NTD, and 16% (SD 25%) in treated HH patients, all significantly higher than the LIR of 1% (SD 22%) measured in ID patients (p<0.05). The LIR was strongly correlated to iron saturation (r=0.41; p<0.01).
Main conclusions Of major interest is the observation that a substantial fraction of oral iron retained in patients with iron overload was not utilized for erythropoiesis. Under circumstances of high transferrin saturation, a part of iron transported from enterocytes into the portal circulation will be non-transferrin-bound iron (NTBI). A part of this NTBI will be available as labile plasma iron (LPI), a form of iron with high redox potential, and the capacity to rapidly cross membranes via transporters and channels. Recently it was shown that in iron-overloaded conditions LPI is almost completely taken up after passage of the liver and this is facilitated by ZIP14 in a non-transferrin-dependent way. (Jenkitkasemwong et al. Cell Metabolism, 2015: 22(1), 138-150). We therefore hypothesize that LPI produced primarily in the portal system (oral dosed iron) is primarily taken up in the liver and that LPI produced elsewhere in the circulation (intravenous dosed iron) may be taken up by other organs as well.
In conclusion, our data is suggestive of the existence of significant hepatic scavenging of NTBI/LPI under iron-overloaded conditions. This could explain the distinct patterns of transfusion-dependent and transfusion-independent iron overload and we suggest that ZIP14 could facilitate this.
van Wijk:Agios Pharmaceuticals: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; RR Mechatronics: Research Funding. van Beers:RR Mechatronics: Research Funding; Bayer: Research Funding; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.